
Materials: Fundamental challenges on the development of materials for integrated CO2 capture and coupled regeneration-methanation
This work package focuses on finding suitable capture and methanation materials, which lowers the energy costs of both capture and methanation and consequently improves their applicability in the Belgian energy system.
CO2 capture makes use of sorbents that become saturated over time when exposed to a CO2 stream. When sorbent regeneration is coupled with methanation, the thermodynamic driving force for sorbent regeneration is provided by the reaction. This increases the overall energy efficiency of the process. To couple these processes, they will need to operate at the same temperature and pressure conditions. This raises important material challenges for both the sorbent and the catalyst.
Mg-based sorbents emerge as a promising candidate for DAC and for CO2 capture from dilute post-combustion flue gases. The regeneration of Mg-based sorbents requires about 100 kJ/mol CO2. Interestingly, Mg-based sorbents begin to slowly release CO2 at the operating temperature of Ni-based methanation catalysts (200-300°C) and are therefore promising candidates for the integrated process. By coupling CO2 capture directly to methanation, the theoretical energy efficiency of the process increases from 83% to 92%. Due to the large deposits of magnesium silicate minerals and its favourable thermodynamics, Mg-based sorbents offer a potential solution for large-scale implementation of coupled regeneration-methanation.
While it is thermodynamically possible to regenerate MgCO3 at 300 °C, fast regeneration at such low temperatures has not been demonstrated. Studies of integrated sorption and methanation are recently emerging, but the performance of the proposed materials is poor and the integrated process has not been quantified. Mg-based sorbents suffer from limited sorption capacities and lose their stability over several sorption-desorption cycles. For DAC and dilute flue gases, the sorbent needs a high sorption capacity and fast sorption kinetics. Development of next generation Mg-based CO2 sorbents is needed, e.g. by fine-tuning the material’s porous architecture and rich chemistry to improve its multi-cycle sorption capacity and sorption-regeneration kinetics.
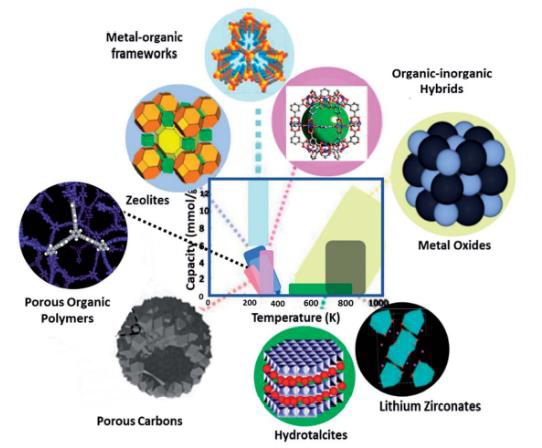
Hydrotalcites are prominent alternatives to MgO due to their significant sorption capacity. Hydrotalcites are naturally occurring layered double hydroxides (LDHs) and can be used as dualfunctional materials when integrated with a suitable catalyst. LDHs exhibit a high multi-cycle stability because of the memory effect, which means they reconstruct back to their original shape after calcination. LDHs can be synthesized in a wide range of chemical compositions (a limited variety are commercially available), have a low cost and are non-toxic. There is need for further modifications to improve the performance of sorption and methanation by improving the porous architecture and chemistry of the materials. Sorbents that regenerate at lower temperatures (e.g. 200°C) can also be considered, when coupled to low-temperature methanation catalysts such as Ru and Pt. The disadvantage of cost-effective lower-temperature capture materials (e.g. activated carbon, zeolites, amine-functionalized materials) is their reduced CO2 affinity. Higher-temperature sorbents such as Ca-based materials only regenerate above 600°C. At this temperature, methanation is thermodynamically unfavourable.
VITO and UGent will leverage their expertise established during the CATCO2RE project to synthesize new Mg-based sorbents and LDHs, with optimized properties for integrated CO2 capture and methanation. When working with structured reactors, the materials are shaped into solid structures using binders (WP.3). This work package will also investigate the impact of the added binders on the performance of capture and regeneration-methanation.
To evaluate the potential of the integrated process, UGent will perform dynamic process simulations using AspenAdsorption. These simulations provide insights in material shaping, reactor operation and the process costs, as a function of material characteristics.
Research questions:
- What combination and ratio of sorbent and catalyst materials can be used for capture and coupled regeneration-methanation? Can we justify the choice of materials and verify the optimal ratio of sorbent-to-catalyst by performing dynamic simulations using a process model?
- Can ideal material characteristics be achieved by modifying synthesis conditions and are the characteristics stable over time? What is the impact of water on the performance?
- What is the impact of the spatial distribution of the active materials (ranging from atomic scale for LDH to macroscale for e.g. multi-layer reactor concept)?
Tasks
T2.1 Development of a suitable sorbent for CO2 capture for dilute flue gases (10% CO2) and DAC (400 ppm CO2)
T2.2 Quantitative proof of concept study for integrated regeneration-methanation
T2.3 Integrated testing of shaped sorbent-catalyst materials for simulated CO2 streams
T2.4 Computational modeling of capture and integrated regeneration-methanation